Follow along as our scientists conduct research at sea
Why does dissolved organic matter matter?

Deep sea dissolved organic matter (DOM) holds almost as much carbon as the atmosphere holds in the form of carbon dioxide.
The difference between the carbon in the atmosphere and carbon held by DOM in the ocean is that DOM is much more dynamic. It can be transformed by both non-living factors (such as light) and living processes (like being eaten by bacteria)—a dynamic factor that is not currently well understood in the deep ocean.
“Carbon dioxide is something we know is rapidly increasing in the atmosphere, so it is important to know how other pools of carbon are changing in response to this aspect of climate change.” — Leanne Powers, post-doctoral researcher
To figure out the role that dissolved organic matter (DOM) plays in the ocean’s carbon cycle and how it might change in the future, we have to understand the basics surrounding it. Where does it come from and how long does it take to degrade?
These are some of the questions Michael Gonsior, lead P.I. on this research cruise, is trying to answer, along with his post-doc Leanne Powers and graduate student Maddy Lahm.
Throughout the cruise, Gonsior, Powers, and Lahm collected almost 100 ten liter water samples to analyze DOM throughout a depth profile. Every 200 meters they collected water all the way down to 4,500 meters—- almost to the sea floor at the Bermuda Atlantic Time-Series.
The water was filtered to be analyzed for optical properties, which is how they quantify fluorescence and chromophoric DO—two aspects of deep-sea DOM that the group is trying to understand.
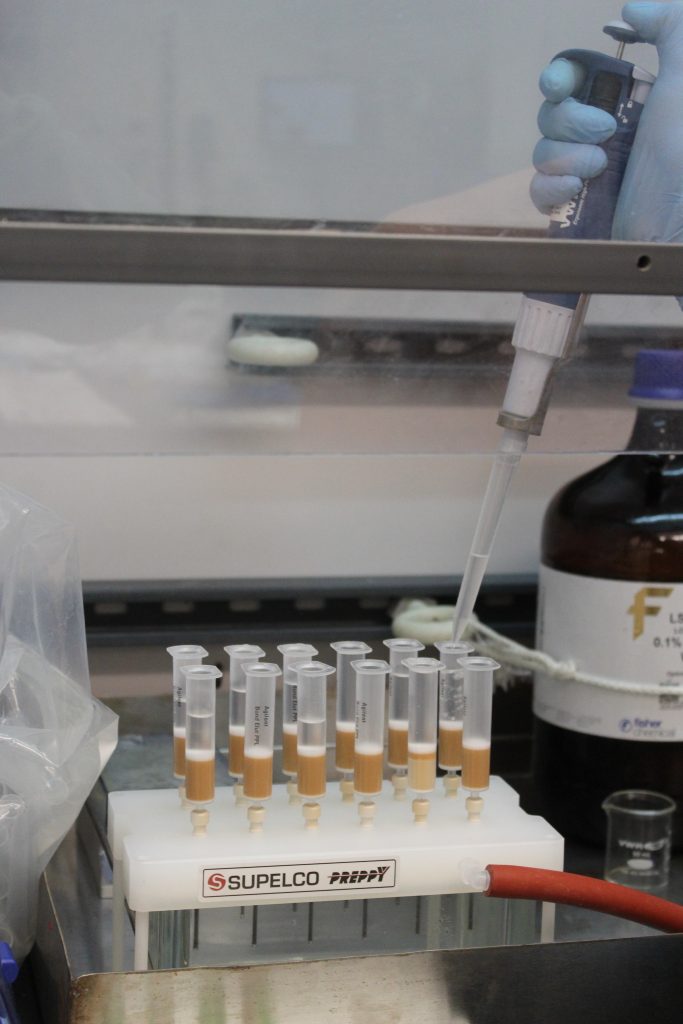
Another analysis done on the boat was a solid phase extraction. In this technique, water is filtered through cartridges containing a resin material that traps organic compounds as they flow through.
Once an entire water sample flows through the cartridges, methanol is inserted into these cartridges and washes out the organic compounds from the resin—essentially making 10 liters of sea water samples into 10 milliliters of methanol with the water’s organic compounds.
The samples are then taken back to a lab and run through a highly specialized mass spectrometer, a machine that is able to give all of the molecular formulas of the thousands of compounds extracted from the water sample.

